Analyze, Assess, Achieve
Our Methodology of Work
We employ a three-step methodology that includes primary analysis, field assessment, and execution to help companies develop actionable and customized projects and plans, formulate solutions to market access challenges, and facilitate successful commercialization throughout Europe and the Middle East.
Primary
Analysis

Field
Assessment

Execution
Service Excellence
Our Services In Europe


Gap Analysis of Evidence
Read MoreAnalyze the relevant clinical and economic evidence per the health authorities expectations and review the literature and guidelines.


Position in Disease Management
Read MoreAssess the position of a new device or therapy in current clinical practice versus the gold standard.


Reimbursement Landscape
Read MoreAnalyze the current coding and payment in the national and regional databases per country.


Roadmap Strategy
Read MoreProvide a roadmap for market access and reimbursement and a comprehensive action plan for implementing the relevant strategies within their respective timelines.


Value Proposition
Read MorePrepare a core value dossier or proposal for innovative health technologies to optimize assessment processes


Interviews with KOLS
Read MorePlan and participate in interviews with healthcare practitioners, hospital executives, and patient advocacy groups.


Early Dialogue With HTA Bodies
Read MorePrepare and participate in early dialogues with local policymakers and health technology evaluation agencies.


Advisory Boards
Read MoreAdvise and organize advisory board meetings with key opinion leaders and experts from different therapeutic areas.


Pricing Strategy
Read MoreDefine the optimal pricing strategy and the required operational actions with payers.


Reimbursement Application
Read MorePrepare reimbursement applications to secure coverage for innovative medical technologies.


Heor Application
Read MoreAdvise and develop a health economic model to generate evidence of the value of new interventions for reimbursement agencies and local healthcare payers.


CED Application
Read MoreSubmit applications for CED programs (coverage with evidence development) for early authorization programs for innovative devices.
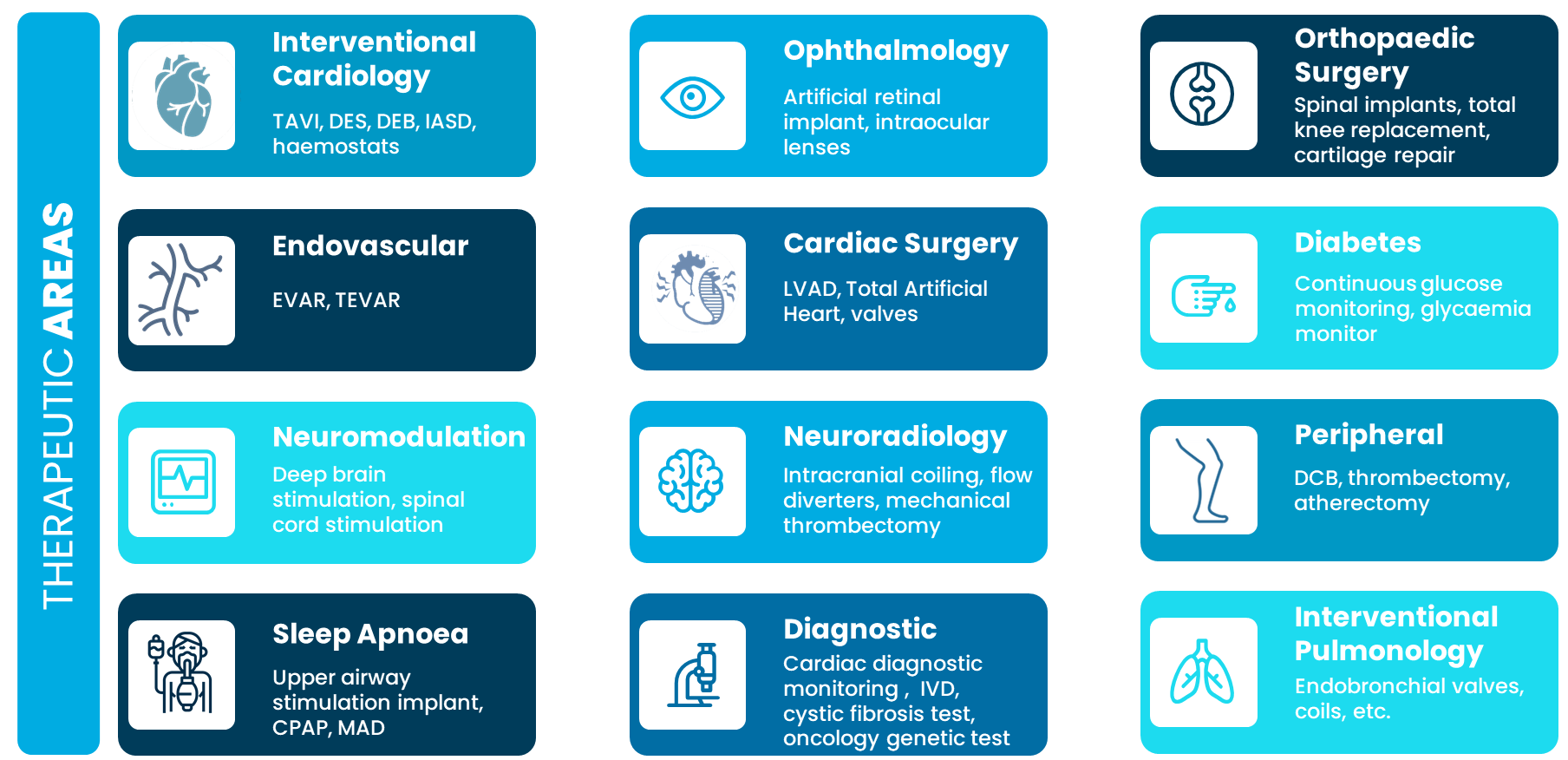
Therapeutic areas
Our Expertise